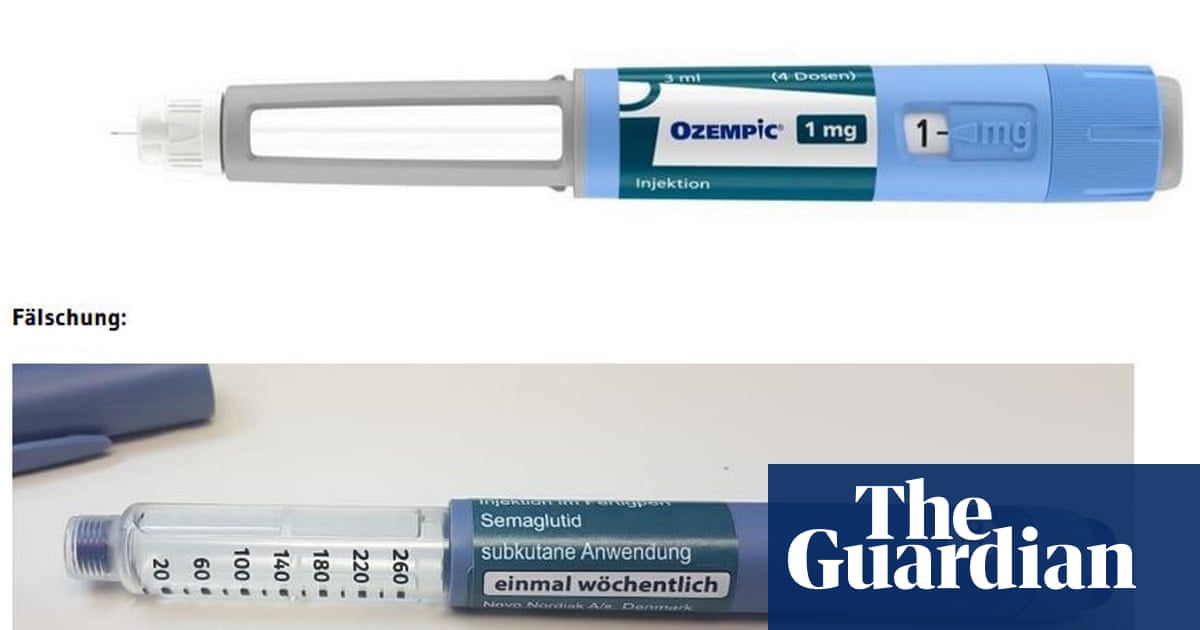
The UK’s regulatory authority for medicines has issued a cautionary notice regarding counterfeit and potentially dangerous weight loss pens. This action was taken after the confiscation of numerous devices being sold by unlicensed vendors.
The pens used by traders to inject drugs are said to contain either Ozempic (semaglutide) or Saxenda (liraglutide), both of which are commonly used for weight loss. However, there are concerns that these pens may also contain unknown substances.
A few individuals who were hospitalized after using the pens have encountered severe adverse reactions, such as hypoglycemic shock and coma. This has prompted health officials to suspect that the pens may contain insulin.
This week, officials in Austria reported that multiple individuals were hospitalized due to injecting fake Ozempic.
The MHRA has advised against purchasing pre-filled pens that claim to contain Saxenda or Ozempic. Instead, it is recommended to seek guidance from a licensed healthcare provider for proper prescription and medication.
Dr. Alison Cave, the chief safety officer at the MHRA, warns against purchasing Ozempic or Saxenda from unauthorized suppliers without a prescription. This greatly raises the chance of receiving counterfeit or non-approved products in the UK.
The medicines regulator has confiscated 369 possibly counterfeit Ozempic pens and received reports of fake Saxenda pens from individuals who obtained them through unauthorized means, such as unregulated websites, without a valid prescription. Before 2023, the regulator had not seized any counterfeit weight loss pens.
According to Cave, the items do not meet rigorous quality and safety requirements, which could potentially jeopardize the health of those who use them. Therefore, he is urging the general public to avoid using any pre-filled weight loss pens purchased online and to instead notify us so that we can conduct an investigation and implement any necessary measures.
Semaglutide and liraglutide are classified as GLP-1 receptor agonists and are prescribed for the treatment of type 2 diabetes and weight management. These medications are administered through injections, commonly referred to as pens or jabs.
In the United Kingdom, Saxenda is approved to be used in conjunction with a healthy diet and exercise as a weight loss treatment. Similarly, Ozempic has been approved for the management of type 2 diabetes in adults, also with the support of a balanced diet and regular physical activity. Although not specifically approved for weight loss in the UK, Ozempic is frequently prescribed off-label for this purpose.
Professor Paul Gately, the CEO of Obesity UK, expressed serious concern about individuals with obesity being targeted by criminals who are obtaining and selling counterfeit weight loss medications. He emphasized the obvious risks to one’s health in such scenarios.
The UK’s health minister, Will Quince, warned that individuals selling illicit medications on the black market posed a major threat to public health and safety.
Quince stated that it is imperative for patients to only use medications like Ozempic or Saxenda when they have a valid prescription from a reliable source, such as their GP or another authorized prescriber. They fully back the MHRA’s efforts to clamp down on these unlawful online providers in order to safeguard patients.
Cave recommended that individuals who believe they are experiencing adverse effects from semaglutide, liraglutide, or any other medication, or suspect they have consumed a counterfeit product, should report it to the MHRA’s Yellow Card program.
Source: theguardian.com
















